Background: Chimeric antigen receptor T-cell therapy (CAR T) has shown significant promise for treating relapsed/refractory hematologic malignancies. Although there is growing literature on immune mediated toxicities, studies examining late effects and survivorship concerns are limited. Sexual health is an important survivorship issue with implications for overall health-related quality of life (HRQOL) and is particularly relevant for young adults (YAs) aged 18-39 years. For YAs, cancer co-occurs with a critical period of physical and psychosocial development during which romantic relationships are formed and physical, psychosocial, and sexual milestones are achieved. No studies have evaluated sexual health among YAs treated with CAR T. To address this knowledge gap, we conducted a mixed methods study to examine sexual health and HRQOL after CAR T among YAs and explore communication about sexual health between YA CAR T recipients and their healthcare providers.
Methods: This was a cross-sectional, single center study conducted at Moffitt Cancer Center. Participants were adults aged 18-39 years treated with CAR T. Participants were asked to complete a one-time survey and an individual semi-structured interview. The survey included short form measures from the Patient-Reported Outcomes Measurement Information System (PROMIS) assessing sexual health (desire/interest in sexual activity, sexual function, sexual satisfaction) and HRQOL (depression, anxiety, fatigue, sleep disturbance, pain interference, global pain, physical function, social function, cognitive function, emotional impact). PROMIS scores were calculated as T-scores with a normative M=50, SD=10, and a minimally important difference (MID) of 5 points. The survey also included study-specific items assessing patient-provider communication about sexual health. For qualitative data collection, a semi-structured interview guide was used to assess the impact of cancer and CAR T on participants' sexual health and HRQOL, as well as patients' experiences with communicating about sexual health with providers, partners, and their broader support system during and after treatment. Preliminary analysis included descriptive statistics for quantitative data in relation to evidence-based thresholds, and a preliminary thematic analysis of qualitative data.
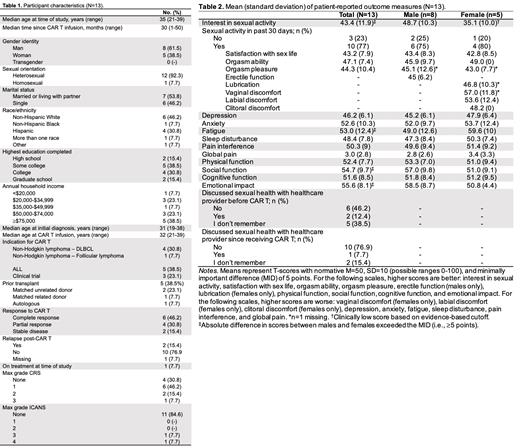
Results: In total, n=13 YA CAR T recipients completed the study survey, and n=10 agreed to participate in individual semi-structured interviews. Participant characteristics are depicted in Table 1. Median age of participants was 35 years (range: 21-39), 61.5% identified as male, and the majority (92.3%) identified as heterosexual. Median time since CAR T infusion was 30 months (range: 1-50). Quantitative data overall and by biological sex are summarized in Table 2. Average interest in sexual activity was low among females (M=35.1, SD=10.0) but not males (M=48.7, SD=10.3). All other average scores were within normal ranges. Several differences in average scores between males and females exceeded the 5-point MID, with female participants reporting less interest in sexual activity, worse fatigue, more impaired social function, and worse emotional impact. Most YAs had not discussed (46.2%) or did not remember discussing (38.5%) sexual health with a healthcare provider before CAR T. Most (76.9%) had not discussed sexual health with a healthcare provider since receiving CAR T. Three major themes emerged from the qualitative data: 1) YAs perceive a lack of awareness of YA sexual health priorities among their healthcare team, 2) There is a lack of communication and information about potential sexual health side effects after CAR T, and 3) Parents as caregivers are a barrier to YAs communicating with their healthcare team about sexual health.
Conclusion: This was the first study to explore sexual health outcomes among YA cancer survivors treated with CAR T. Findings highlight a need for improved patient-provider communication regarding sexual health and more publicly available educational resources. Providers may prioritize one-on-one discussions with patients and limit parent involvement when discussing sexual health needs. As the population of CAR T survivors continues to grow, it is imperative to examine patient reported outcomes and late effects to improve survivorship outcomes.
Author Note: AB & VIG share first authorship. LO & RF share senior authorship.
Disclosures
Oswald:Pentecost Family Myeloma Research Center: Research Funding; H. Lee Moffitt Cancer Center and Research Institute: Current Employment; Dana-Farber Cancer Institute: Consultancy. Faramand:Kite: Research Funding; Gilead: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal